Glycans are present in all living cells and organisms, and their crucial roles and functions are as diverse as acting as structural components for cell integrity or in regulating physiological and pathological processes, e.g., signalling, cell-cell communication, immune-recognition, host-pathogen interactions, protein folding and quality control.
Although it is recognised by the scientific community that glycans represent a largely undiscovered resource for biological functions as well as for novel therapeutic opportunities, their study and the study of glycan-binding proteins has been hampered by several challenges. Among these are the following:
1) glycans have highly heterogeneous sequences;
2) are difficult to isolate from natural sources in sufficient amounts for characterization by conventional methods; and
3) the glycan-protein interaction is in general of low affinity.
But these studies are being revolutionised by the advent of the highly sensitive and miniaturised glycan microarray technology, whereby sequence-defined glycan probes are robotically printed on solid supports as microspots.
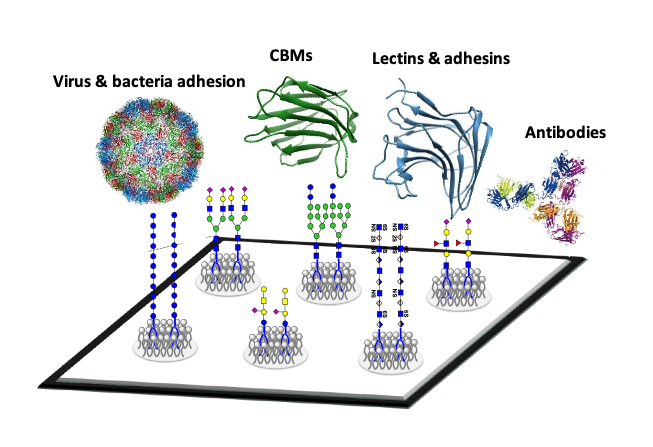
Glycan microarrays have proven to be powerful high-throughput screening tools for discovering glycan ligands and their function and evaluating specificities of glycan-binding proteins; also for assignment of functional glycan ligands in pathogen-host interactions.
Despite the great advances in the glycan microarray field, its continuous development is essential, for example, for studies of microbe-host interactions or to target specific mammalian or microbial glycomes.
As the desired glycans that are relevant in those particular biological contexts are unavailable, it is critical to generate microarrays from biologically relevant ligand-bearing glycoconjugates, in order to reveal the oligosaccharide ligands they harbour, so that these can be isolated and characterised.
This is the concept of our designer microarray approach.