We work to understand the glycan-protein interactions in the context of natural glycans.
Glycans have essential biological functions: they can mediate cell-cell, cell-nutrient, host-pathogen interactions. This is why alterations in the glycosylation of proteins and lipids are associated with several diseases. The study of these interactions is crucial for discovering novel biomarkers and developing glycan based diagnostic/therapies.
Our primary goals are:
1) To identify function glycan epitopes for proteins at a glycomics scale,
2) Discover glycan-binding proteins and
3) Characterize glycan structure and function in health and disease.
We use an integrative approach combining glycan microarrays and structural biology to unravel glycan ligand-protein complexes and understand the molecular determinants of glycan recognition.
Our research highlights are:
1) Targeting plant cell wall polysaccharides recognition by cellulolytic bacteria
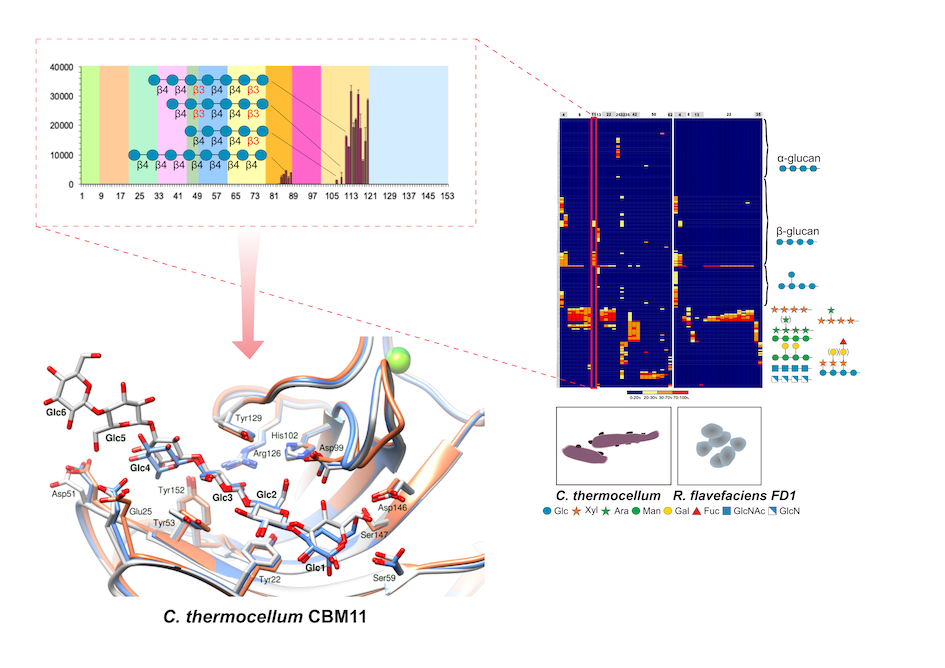
The plant cell-wall is constituted by structurally diverse polysaccharides whose biodegradation is a crucial process for life sustainability. Cellulolytic microorganisms are highly efficient in this process by assembling modular architectures of carbohydrate-active enzymes (CAZymes) with appended non-catalytic carbohydrate-binding modules (CBMs). Carbohydrate microarrays offer high-throughput and sensitive tools for uncovering carbohydrate-binding specificities of CBMs, which is pivotal to understand the function of these modules in polysaccharide biodegradation mechanisms (The Royal Society of Chemistry, 2018, 43).
Recently, we have detailed the molecular determinants for the carbohydrate recognition of Clostridium thermocellum family 11 carbohydrate-binding (CtCBM11) (THE FEBS JOURNAL 2020; 287, 2723). CtCBM11 is an archetypal example of carbohydrate‐binding modules binding to mixed‐linked glucans with a preference for β1,3‐1,4‐glucans. The analysis of this interaction by carbohydrate microarrays, nuclear magnetic resonance (NMR) and X‐ray crystallography has established the molecular basis of this unique recognition mechanism, including the distinction between linear and twisted mixed‐linked β‐glucans, inspiring the design of new biomolecules with applications in health and agriculture.
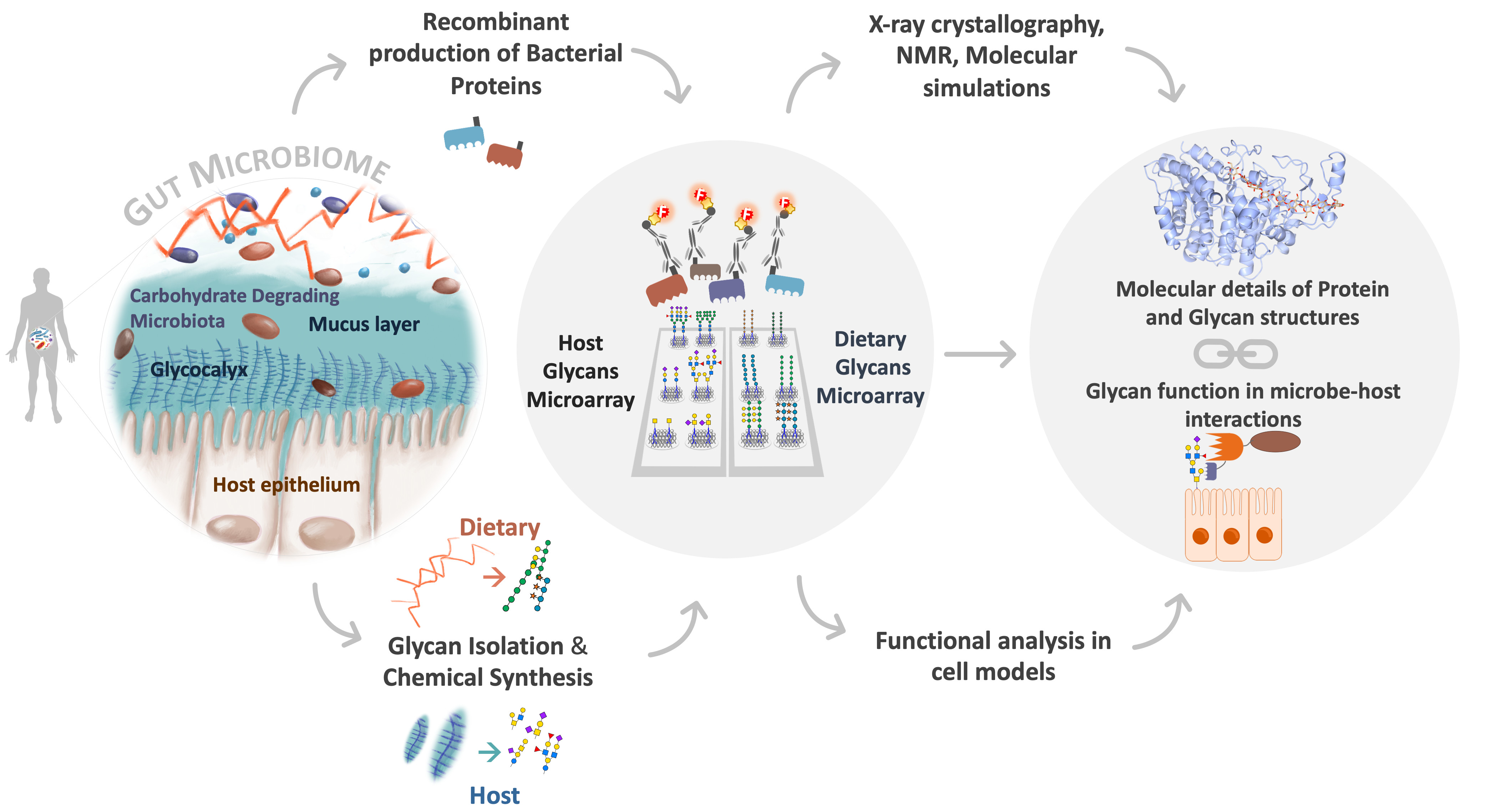
2) Glycan-mediated host-microbiome interactions
The project GLYCOINTERACT aims to understand at a molecular level the glycan-human microbiome interaction. The gut resident microbiome interacts with diverse and complex glycan structures present in the mucosal layer of the intestinal epithelia. Whereas commensal bacteria use mucosal glycans as nutrients with health benefits for the host, pathogenic bacteria use these to initiate infection and breach the surface. Alterations to the mucosal barrier, associated with an imbalance in the microbiome (dysbiosis), are attributed to the onset of several diseases.
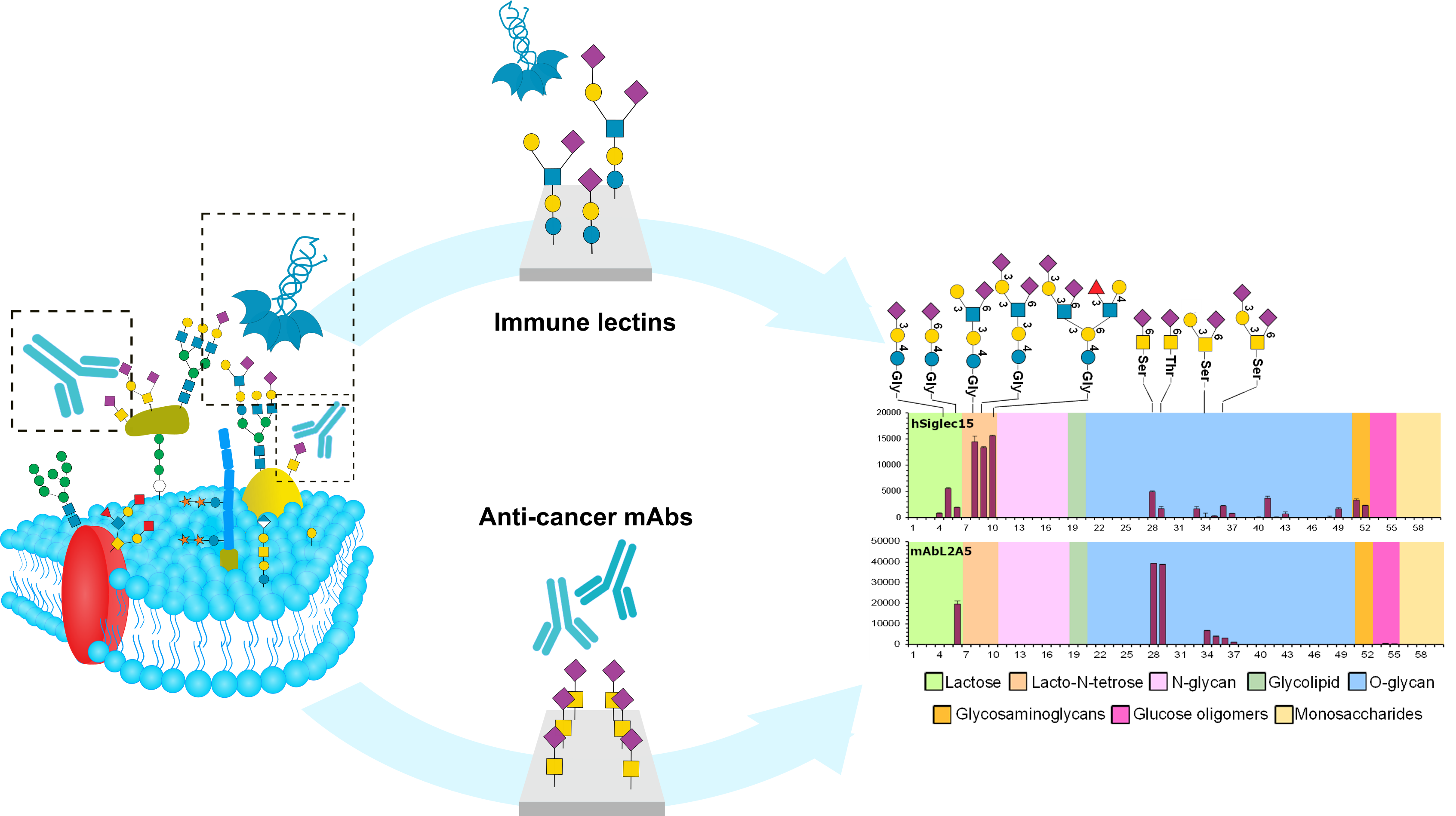
3) Glycan ligand discovery and specificity assignment for immune-lectins and anti-cancer antibodies
The recognition of glycans by effector proteins of the immune system is critical to mediate signalling events in disease, including in tumour immune-invasion and progression. We are applying recently developed microarrays of structurally-defined glycans found in normal and tumour cells to identify the glycan ligands and assign specificity of immune-lectins and therapeutic antibodies.